Confirmation of simulation prediction
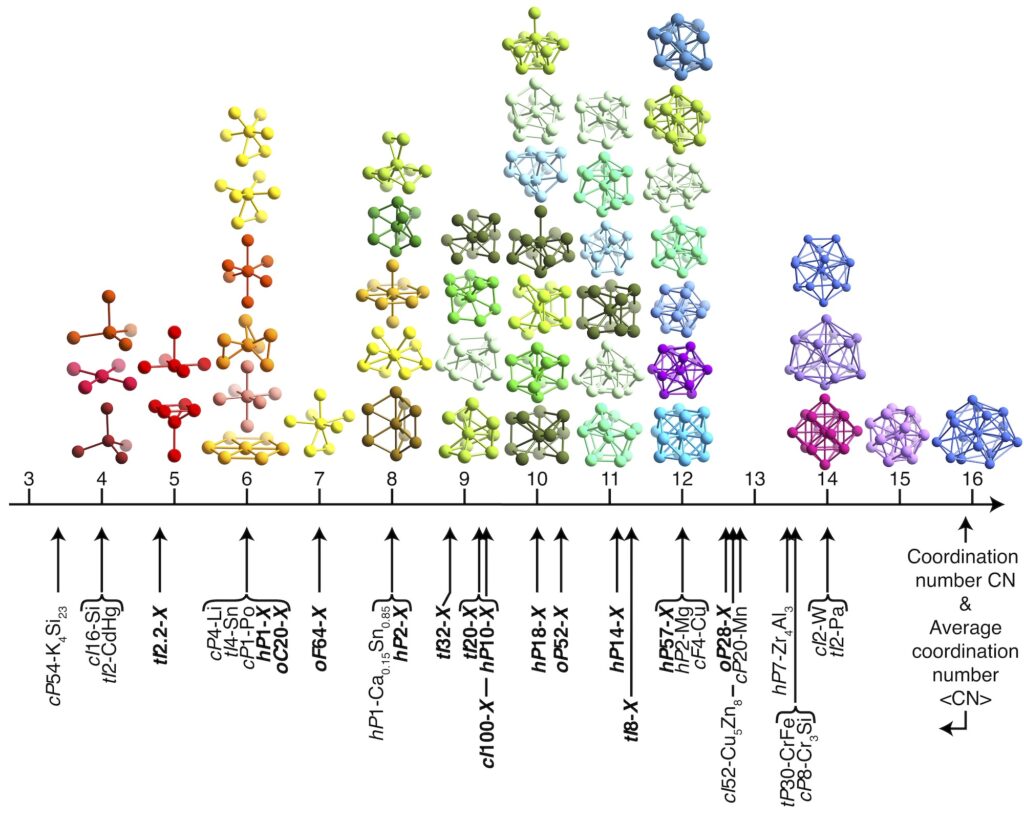
The tetrahedral geometry is ubiquitous in natural and synthetic systems. Regular tetrahedra do not tile space, which makes understanding their self-assembly behavior a formidable challenge. In 2009, simulations of hard tetrahedra —that is particles with the shape of a regular tetrahedron, interacting only by excluded volume interactions— discovered a dodecagonal quasicrystal stabilized by entropy alone. But while this quasicrystal forms robustly and reproducibly in simulation, it competes with periodic approximants and cannot be the thermodynamic ground state in the limit of infinite pressure. In this limit, the densest packing will eventually prevail, which is a simple (in comparison) dimer crystal.
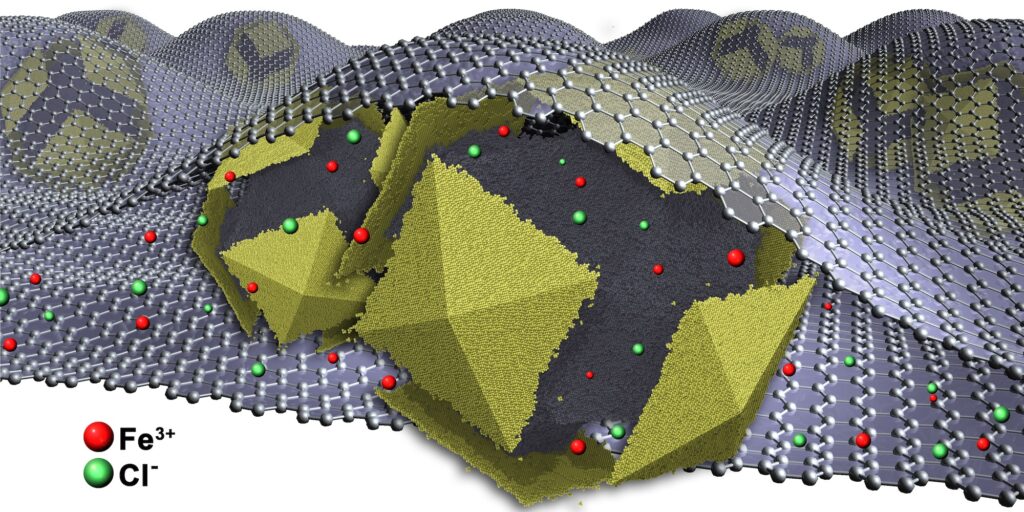
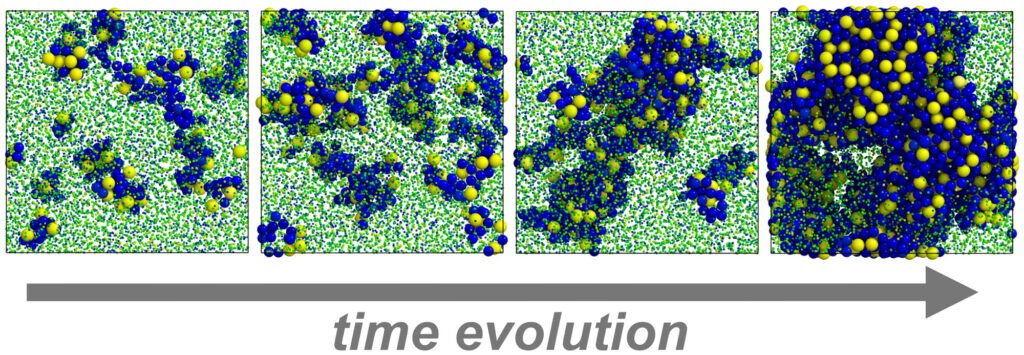
Finally, after 14 years, our simulation predictions are confirmed. Yi Wang from the group of Xingchen Ye at Indiana University (USA) experimentally realized multiple phases of tetrahedron colloids where vertex sharpness, surface ligands, and the self-assembly environment play key roles in the formation of the quasicrystal and the dimer crystal. Our colleagues at the Institute of Micro- and Nanostructure Research at FAU resolved the complex three-dimensional structure of the quasicrystal by a combination of electron microscopy, tomography, and synchrotron X-ray scattering. The joint findings demonstrate the predictive power of computer simulations as well as the importance of accurate control over nanocrystal attributes and the assembly method to realize increasingly complex nanopolyhedron supracrystals.
Read about the research here:
Yi Wang, Jun Chen, Ruipeng Li, Alexander Götz, Dominik Drobek, Thomas Przybilla, Sabine Hübner, Philipp Pelz, Lin Yang, Benjamin Apeleo Zubiri, Erdmann Spiecker, Michael Engel, Xingchen Ye
Controlled Self-Assembly of Gold Nanotetrahedra into Quasicrystals and Complex Periodic Supracrystals
Journal of the American Chemical Society 145, 17902 (2023)