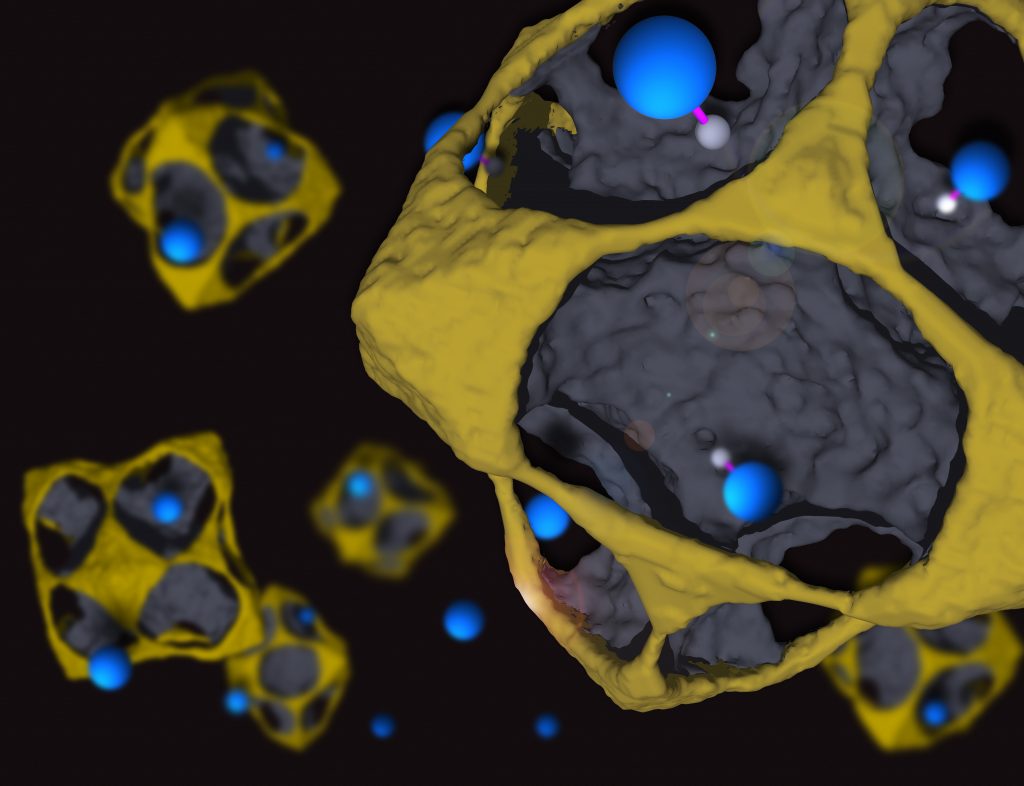
Controlling nanocrystal shapes
In a recent ACS Nano publication, Alberto Leonardi proposes an etching synthesis method for controlling the shape of core-shell nanocrystals:
“The application of nanocrystals as heterogeneous catalysts and plasmonic nanoparticles requires fine control of their shape and chemical composition. A promising idea to achieve synergistic effects is to combine two distinct chemical and/or physical functionalities in bimetallic core@shell nanocrystals. Although techniques for the synthesis of single-component nanocrystals with spherical or anisotropic shape are well-established, new methods are sought to tailor multicomponent nanocrystals. Here, we probe etching in a controlled redox environment as a synthesis technique for multicomponent nanocrystals. Our Monte Carlo computer simulations demonstrate the appearance of characteristic non-equilibrium intermediate microstructures that are further thermodynamically tested and analyzed with molecular dynamics. Convex platelet, concave polyhedron, pod, cage, and strutted-cage shapes are obtained at room temperature with fully coherent structure exposing crystallographic facets and chemical elements along distinct particle crystallographic directions. We observe that structural and dynamic properties are markedly modified compared to the untreated compact nanocrystal.”
Read about it here:
Particle Shape Control via Etching of Core-Shell Nanocrystals
A. Leonardi, M. Engel
ACS Nano 12, 9186-9195 (2018)